Treating Cancer by Spindle Assembly Checkpoint Abrogation: Discovery of Two Clinical Candidates, BAY 1161909 and BAY 1217389, Targeting MPS1 Kinase.
Schulze, V.K., Klar, U., Kosemund, D., Wengner, A.M., Siemeister, G., Stockigt, D., Neuhaus, R., Lienau, P., Bader, B., Prechtl, S., Holton, S.J., Briem, H., Marquardt, T., Schirok, H., Jautelat, R., Bohlmann, R., Nguyen, D., Fernandez-Montalvan, A.E., Bomer, U., Eberspaecher, U., Bruning, M., Dohr, O., Raschke, M., Kreft, B., Mumberg, D., Ziegelbauer, K., Brands, M., von Nussbaum, F., Koppitz, M.(2020) J Med Chem 63: 8025-8042
- PubMed: 32338514
- DOI: https://doi.org/10.1021/acs.jmedchem.9b02035
- Primary Citation of Related Structures:
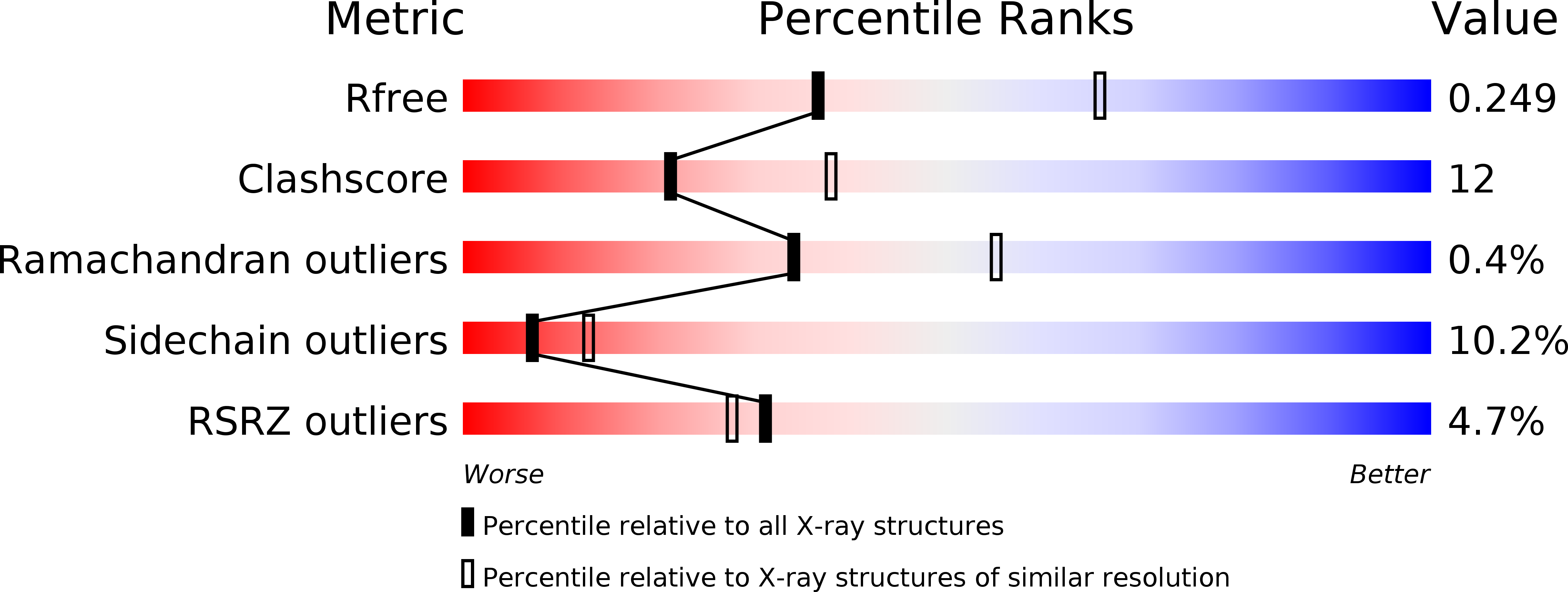
6TN9, 6TNB, 6TNC, 6TND - PubMed Abstract:
Inhibition of monopolar spindle 1 (MPS1) kinase represents a novel approach to cancer treatment: instead of arresting the cell cycle in tumor cells, cells are driven into mitosis irrespective of DNA damage and unattached/misattached chromosomes, resulting in aneuploidy and cell death. Starting points for our optimization efforts with the goal to identify MPS1 inhibitors were two HTS hits from the distinct chemical series "triazolopyridines" and "imidazopyrazines". The major initial issue of the triazolopyridine series was the moderate potency of the HTS hits. The imidazopyrazine series displayed more than 10-fold higher potencies; however, in the early project phase, this series suffered from poor metabolic stability. Here, we outline the evolution of the two hit series to clinical candidates BAY 1161909 and BAY 1217389 and reveal how both clinical candidates bind to the ATP site of MPS1 kinase, while addressing different pockets utilizing different binding interactions, along with their synthesis and preclinical characterization in selected in vivo efficacy models.
Organizational Affiliation:
Research & Development, Pharmaceuticals, Bayer AG, 13353 Berlin, Germany.